FDA-Approved, Single-Shot RSV Vaccine for Infants Offers Full-Season Protection
A new FDA approval could revolutionize the prevention of RSV in infants and small children.
By
Patrick Campbell
| Published on June 12, 2025
4 min read
Credit: Adobe Stock
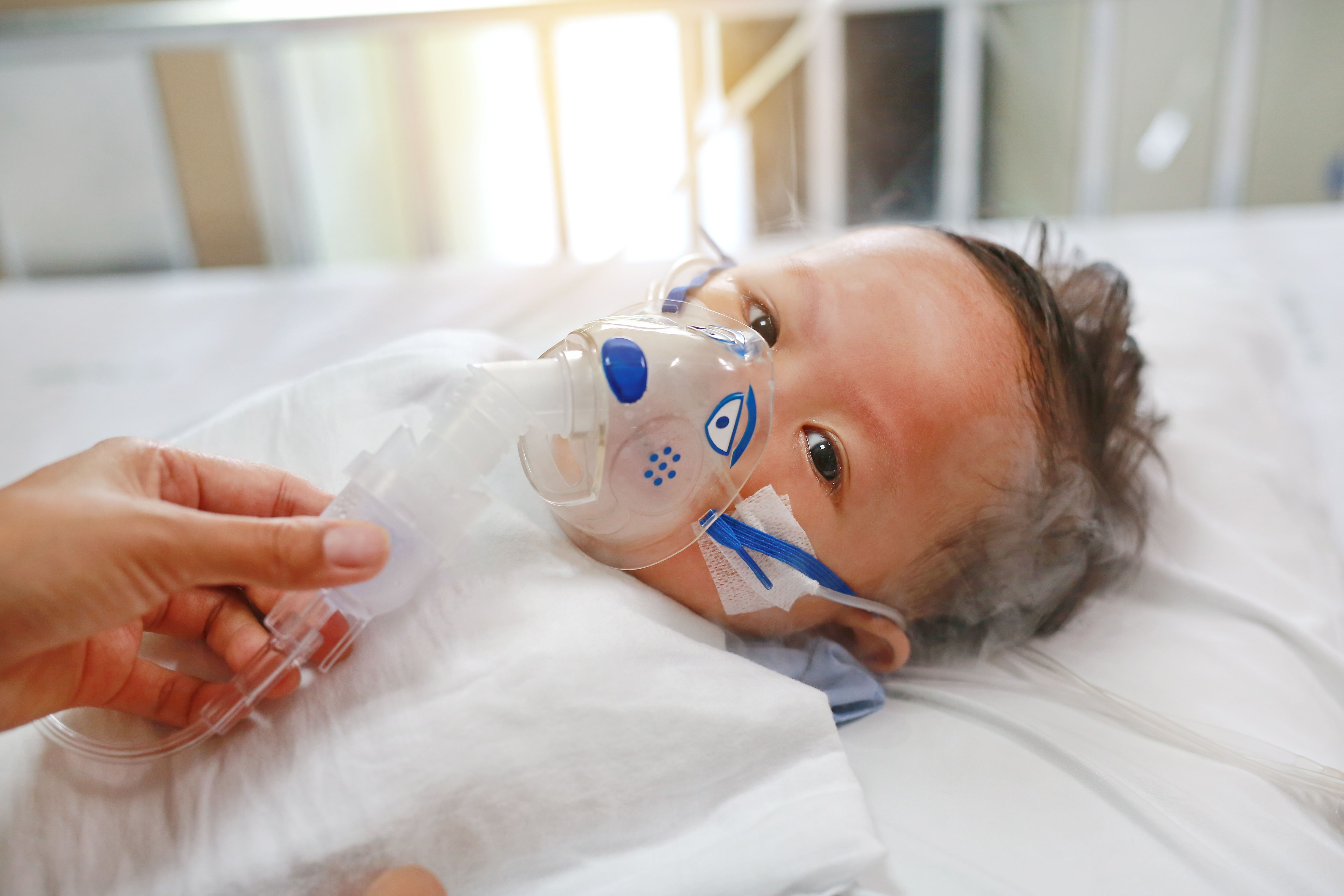
The U.S. Food and Drug Administration has approved a new monoclonal antibody injection to protect infants from respiratory syncytial virus, or RSV, during their first season of exposure. Clesrovimab (Enflonsia) is expected to be available before the 2025-2026 RSV season begins, according to Merck, the drug’s manufacturer.
The June 9, 2025, approval follows encouraging results from a large clinical trial and adds a powerful new tool for preventing a virus that is a leading cause of illness and hospitalization in young children.
A Major Threat to Infant Health
RSV is the most common cause of hospitalization in infants under 1 year old in the United States, according to the Centers for Disease Control and Prevention. Each year, the virus leads to an estimated 58,000 to 80,000 hospitalizations and up to 300 deaths among children under 5 years old. It can cause severe lower respiratory infections like bronchiolitis and pneumonia, particularly in babies born prematurely or with other health risks.
The virus spreads easily and follows a seasonal pattern, typically peaking between fall and early spring. Infants born during or just before RSV season are at the highest risk for complications.
One Shot for Full-Season Protection
Clesrovimab is designed to protect infants from RSV throughout the entire five-month season with a single intramuscular dose. Unlike traditional vaccines, which rely on the body to build up immunity, this monoclonal antibody provides immediate passive protection.
The preventative option was tested in the phase 2b/3 CLEVER trial, which enrolled 3,632 healthy preterm and full-term infants. Participants were randomized 2-to-1 to receive either a single dose of clesrovimab or a placebo.
The study results showed the following:
- A 60.5% reduction in medically attended RSV-related lower respiratory infections
- An 84.3% reduction in RSV-related hospitalizations
- A 90.9% reduction in hospitalizations due to severe RSV infection
- A 91.7% reduction in severe medically attended RSV infections
The results were consistent at both the five- and six-month follow-up points, according to data presented by Merck at IDWeek 2024.
A New Approach to RSV Prevention
Clesrovimab works differently from existing RSV preventives. It targets a unique site on the RSV protein known as site IV, which is present in both pre-fusion and post-fusion forms of the virus. This contrasts with other monoclonal antibodies, such as nirsevimab, which target a different viral site.
Laboratory studies have shown clesrovimab can neutralize more than 96% of RSV A and B strains. Because of its extended half-life, it remains active in the body longer than older monoclonals. It also uses non-weight-based dosing, meaning all infants can receive the same dose, simplifying administration.
Looking Ahead
With clesrovimab’s approval, pediatricians and parents now have another safe, effective way to reduce the risk of serious RSV infections. For infants born during RSV season, the shot can be given starting at birth. For others, it can be administered before their first exposure.
As RSV continues to pose a seasonal threat, health officials say tools like Enflonsia will be critical to protecting the youngest and most vulnerable children from hospitalization and long-term complications.
