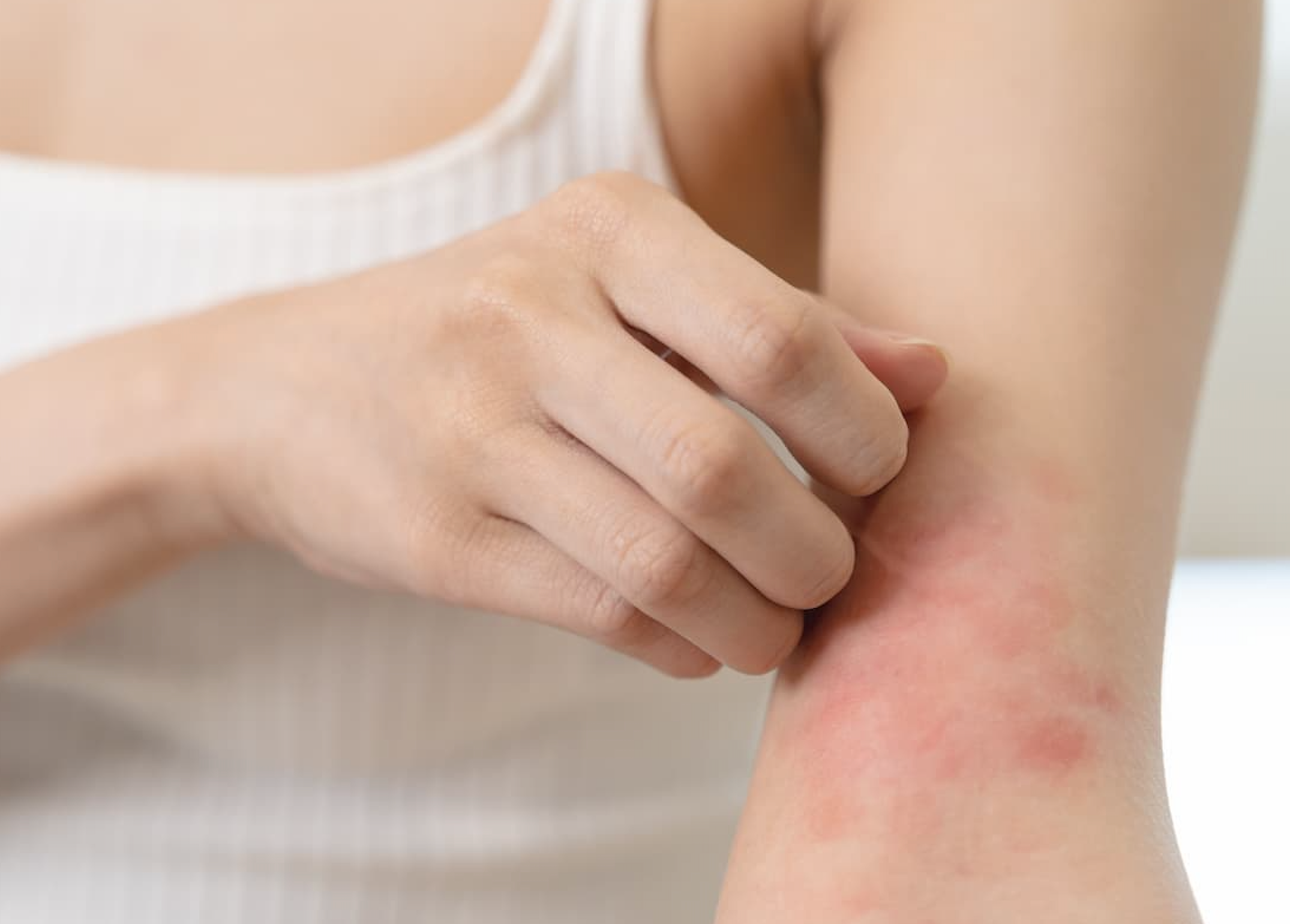
FDA Approves First-Ever Treatment for Chronic Hand Eczema in Adults
The FDA has approved delgocitinib (Anzupgo) cream — the first treatment specifically for adults with moderate to severe chronic hand eczema.
By
Lana Pine| Published on July 24, 2025
2 min read
If you live with chronic hand eczema (CHE) — the kind that sticks around for months, comes back often and affects your daily life — there’s finally some long-awaited good news. The U.S. Food and Drug Administration (FDA) has approved delgocitinib (Anzupgo) cream (20 milligrams/gram) for adults with moderate to severe CHE who haven’t had success with, or can’t use, topical steroids.
CHE affects roughly 1 in 10 adults worldwide, and until now, there were no treatments specifically approved in the U.S. for this common yet often overlooked skin condition.
“Anzupgo is a good example of how we transform a real need in the market into medicines that can help make a difference for people living with serious skin diseases such as CHE,” said Christophe Bourdon, CEO of LEO Pharma.
Delgocitinib cream is a steroid-free topical medication that works by calming the overactive immune response that causes hand eczema symptoms. It blocks the JAK-STAT pathway, a key player in inflammation, making it the first pan-JAK inhibitor cream of its kind approved in the U.S.
People with CHE know the toll it can take — constant itching, painful cracks, swelling and even blisters that can interfere with work, hobbies and everyday tasks. Studies show that up to 70% of patients with severe CHE struggle with daily activities, and many report emotional stress, sleep issues and job limitations due to the disease.
The FDA approval of delgocitinib follows similar approvals in Europe, the U.K., and the United Arab Emirates. LEO Pharma, the company behind the cream, has also expanded its U.S. operations to support the rollout, emphasizing just how significant this launch is.
“The approval of Anzupgo reinforces our commitment to investing in difficult-to-treat skin conditions to deliver new treatments to patients where the need is greatest,” said Bourdon.

