Several OTC Eye Drops Recalled Over Manufacturing Concerns
A voluntary recall has been issued for several over-the-counter eye drop products due to manufacturing issues that could affect their safety.
By
Lana Pine
| Published on May 14, 2025
2 min read
Credit: Adobe Stock/Dragana Gordic
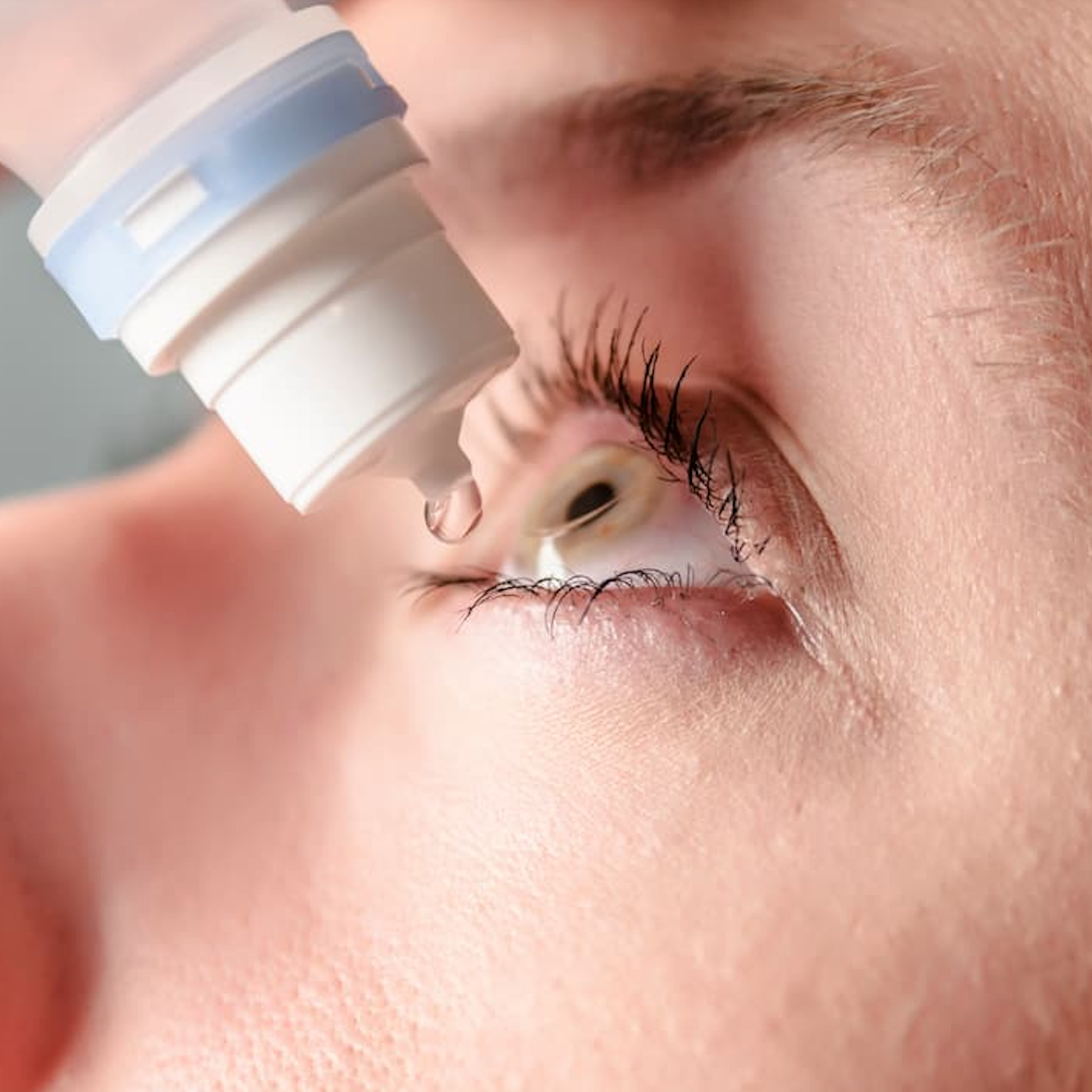
Patients using over-the-counter eye drops from BRS Analytical Service, LLC, including artificial tears and other lubricating products, are being urged to stop using them immediately due to potential manufacturing problems that could affect product safety and quality.
AvKARE, the company’s distributor, has issued a voluntary recall at the consumer level, meaning the eye drops should no longer be used by anyone who has them at home. The recall comes after the U.S. Food and Drug Administration (FDA) found problems during a routine audit that suggest the products may not meet required current Good Manufacturing Practice (cGMP) regulations.
While no specific health risk has been confirmed yet, the company and the FDA have stated that it is not possible to rule out patient harm due to possible quality issues. Patients are advised to stop using the recalled products immediately.
The recall includes the following over-the-counter eye drops, distributed under National Drug Code (NDC) numbers:
- Artificial Tears Ophthalmic Solution (NDC# 50268-043-15)
- Carboxymethylcellulose Sodium Ophthalmic Gel 1% (NDC# 50268-066-15)
- Carboxymethylcellulose Sodium Ophthalmic Solution (NDC# 50268-068-15)
- Lubricant Eye Drops Solution (NDC# 50268-126-15)
- Polyvinyl Alcohol Ophthalmic Solution (NDC# 50268-678-15)
These products were shipped between May 26, 2023, and April 21, 2025.
A full list of product codes is available here.
If you have any of these products in your home, stop using them immediately, check the NDC number on the packaging to confirm whether the product is affected, and return the product or dispose of it safely.
Contact your health care provider if you have experienced any issues such as eye irritation, infection or changes in vision after using these products.
Consumers are also encouraged to fill out the “Quantity to Return” section on the official recall notice and fax it to 931-292-6229 or email it to customerservice@avkare.com, even if they no longer have the product.
For additional information, contact customerservice@avkare.com.

